Happy birthday to stem cells. Now, make a wish.
Call it an early New Year’s resolution. On any given day of the week, there are dozens of events in Madison worth attending. Take Tuesday, November 18, for example. On that date one could choose from over a dozen different free seminars on topics ranging from digital publishing to solar-powered electric cars. And they were all within a 4-mile radius! It’s supremely easy to feed your brain here in Madtown. So I’ve made myself a promise: if there is a science, patent law, or biotech business event that is after normal working hours (sorry, clients come first), preferably free, and it poses no logistical conflicts on the home front (sorry, my daughter’s dance recital wins out), then I’m there. Free food? Even better.
Thus I found myself heading to the Overture Center on a chilly November evening, striding amicably past a half-dozen protesters carrying signs proclaiming “People for the Ethical Treatment of Embryos”. The event that night was organized by the Wisconsin Academy of Arts and Letters and was part of a two-day series celebrating the ten-year anniversary of the first research publication by UW-Madison professor James Thomson reporting the isolation and culturing of human embryonic stem cells.
There were some heavy hitters present. At least in spirit, anyway. The night opened with remarks by Gov. Jim Doyle - prerecorded, since he was at a Governor’s summit on environmental policy being held in California. He emphasized the impact of stem cells on the biotech industry in Wisconsin. Biotech currently employs 32,000 people in the state and accounts for $9B in revenue, but Doyle’s office stated that regenerative medicine and stem cells is projected to account for a $500B segment in the next 10 years. (Although, really, can we take any market projections at face value right now, given the evolving global recession?) Bioethicist and UW law professor R. Alta Charo was scheduled to speak, but could not attend – because she had been called to Washington, D.C. to serve on President-elect Obama’s transition team. Secretary of Commerce Dick Leinenkugel introduced Dr. Thomson, summarizing Thomson’s biography: undergraduate degree in biophysics, D.V.M. and Ph.D. from the University of Pennsylvania, board certification as a veterinary pathologist, and recently elected to the National Academy of Sciences. He also achieved a pop culture nod from Time Magazine highlighting him as on of the 100 most influential people. In the world, that is.
As Jamie Thomson took the stage, he quietly opened with, “Well, it’s been quite a 10 years, and one of the things that still amazes me is that people are still gracious enough to come and hear me talk.”
The humility doesn’t appear to be for show. Thomson has spent a tremendous amount of time and energy talking about stem cell biology to the public in the past decade, but when it comes to interviews on his personal perspectives and style, he is most famous for his shyness. So not surprisingly, his talk focused squarely on the science.
Let’s start with the end, and work ourselves back to the beginning. Understanding stem cells is straightforward, in Thomson’s view. “Human embryonic stem cells are normal cells that can divide without limit and can develop into any of the cells of the body”, he explained.
In a nutshell, that’s it. They are a relentlessly inexhaustible blank slate. Personally I find it amazing that this irony isn’t remarked upon more often; yes, the original structure of a blastocyst is destroyed in order to establish a cell line, but the cell culture derived from it lives on indefinitely. His group started culturing five different embryonic stem cell lines in 1998. They still exist in Petri dishes and freezers today, are still sent to hundreds of laboratories all over the world, still divide happily, still can be coaxed into forming heart cells or skin cells or neurons.
Why is this a big deal? Because prior to Thomson’s work in 1998, it was thought to be impossible. If you wanted to study human cardiomyocytes (heart cells), you had to harvest a heart, or use cultured heart cells derived from a harvested heart, which sometimes did not remain terrifically heart-like. If you wanted to study neurons, you had some dissection to do. And so forth. Peering back in time to see molecular events during development of human heart cells was difficult to impossible; that window in time remained dark and inaccessible.
Thomson, of course, did not arrive at his embryonic stem cell technique out of nowhere. He had studied precursor research carefully, such as research on teratocarcinomas (the cancer equivalent of stem cells) that had been conducted in mice since the 1950s. In 1983, Thomson studied a technique called nuclear transfer with his advisor at the Wistar Institute, Davor Solter. But even when embryonic stem cell culture was achieved in other species, the prevailing thought was that it couldn’t be done in humans.
Thomson wasn’t convinced.
His focus was on embryo development, and he soon tired of studying mouse embryos and supposing that what was seen was similar to human embryo development when some patterns were so clearly different. So in 1991, he came to UW to conduct embryo development research at the Primate Center. Working on a New World monkey (common marmoset) and an Old World monkey (rhesus), he found that he was able to harvest embryos – but it was a laborious, depressing process. No one talks about preventing abortion in monkeys, but suffice it to say that if research monkey mothers could make their case known, they’d be strong supporters of embryonic stem cell culture research. Far preferable to scientists and monkeys alike would be the ability to maintain tissue-culture harvestable material. In that case you could harvest once, maintain the cells forever, and spend more time actually researching development than doing what researchers informally called “embryo flushes” (using fluid to dislodge embryos from the primate uterus).
So, Thomson and his research group did just that. Less famous than his human stem cell research articles were two 1995 articles from his group on primate stem cell research, one for marmosets and one for rhesus monkeys. It was only with this proof-of-concept work in hand that he could begin work to isolate human embryonic stem cells.
But, Thomson had no desire to plunge ahead with his research plans in absence of careful consideration. So he consulted with bioethicists Alta Charo and Norman Fost before doing any of his experiments. The embryos that were used for the research were ones that had been generated by in vitro fertilization clinics – extras from parents who had already implanted all the embryos they wanted to use for a pregnancy, and which would never be used for any other purpose. Slated to be destroyed, they were used by Thomson’s group, which modified the primate embryonic stem cell technique for the human embryo material – thereby deriving the now-famous cell lines. (Even ten years later, the decision of what to do with extra IVF-generated embryos remains a difficult one with limited options available to parents, as the New York Times reports.)
The impact of the Thomson laboratory’s 1998 research article was immediate. The paper was published in the journal Science on a Friday, and Thomson was asked by Sen. Arlen Specter (R-PA) to testify before congress the following week. “[The issue of stem cells] wasn’t politically partisan at the time of publishing”, Thomson noted.
But the research published in 1998 was just the beginning of the scientific story. One year before Thomson published the original human embryonic stem cell research, Ian Wilmut’s group at the Roslin Institute in Scotland had shown that they could substitute the nucleus from one sheep into an enucleated egg of another sheep to create the world’s first cloned mammal, dubbed Dolly. “The real take-home message of Dolly was not that somatic cell transfer would occur, but rather that the differentiation process could go backwards”, Thomson said.
To plant biologists like myself, this is a tad underwhelming. Totipotent plant cells have been known for decades, and cloning a plant requires no specialized expertise – my grandmother, bless her, had an entire spare bedroom devoted to her cloned African violets. But mammals are less malleable, and Dolly had gotten Thomson and everyone else in the developmental biology field thinking. If development could really go backwards, why not coax any cell, not just an embryo cell, back into a stem culture state?
It took years to hone the methods, but they did it. In November 2007, techniques for producing induced pluripotent stem cells (iPS), independently discovered by Thomson’s group and the research group of Shinya Yamanaka of Kyoto University, were published on the same day. It will still take considerable time to determine whether induced stem cells truly have all the hallmark characteristics of embryonic stem cells, but early indications are good.
Thomson spent the remainder of his lecture on practical applications of human embryonic stem cells and iPS cells. In brief, they can be broken down into stem cells for the sake of research, or stem cells for direct use in patients.
In the first case, stem cells are used as test material to more accurately determine the safety of new drugs, for example, or to find drugs that act on specific cell types. Right now such pre-clinical testing is done using a messy approximation of animals, test tube approaches, and small-scale human studies reserved only for the most promising drug candidates. It’s not good enough. Some potential drugs that appear promising are found to be unexpectedly toxic. Often this is discovered too late in the game, after millions have been poured into R&D. This scenario – using iPS cells to test or discover new drugs - is where Thomson sees the most immediate promise. “One of the ways you have to give discoveries to the world is through the commercialization process”, he reasoned, and towards that end he has founded Cellular Dynamics International, Inc. CDI announced in November 2008 that it has raised over $18M in financing.
In the second scenario, stem cells are used as potentially unlimited sources of transplantation therapies. Doctors could treat victims of neurological accident or disease, for example, by supplying stem cells to create new neurons; diabetics might receive a dose of stem cells delivered to the pancreas to generate new islets of Langerhans. The more fantastic projections (though perhaps not so outlandish after all) suggest tissue or even organ regeneration on an as-needed basis. Delivering a sobering dose of reality, however, Thomson stated, “This has been over-sold in terms of timeline”, and cautioned in several examples (cardiac stem cell transplantation, motor neuron transplantation to treat Parkinson’s disease) that if the underlying reason for the death of the patient’s original cells is not addressed, then stem cell transplants are likely doomed to the same fate. He also warned that when introducing happy-to-proliferate cells, there is a very real possibility of causing cancer. Depending on the application, if one isn’t careful, the cure could be worse than the disease. Type I diabetes, for example, is a serious and life-limiting disease, but if treated properly can be controlled. Pancreatic cancer, however, can lead to an end that is nasty, brutish, and short. Great care is warranted when determining what avenues are best to pursue.
As the lecture wound down into a Q&A session, there were remarkably few questions from the audience. Thomson was kindly and approachable, but his sheer intensity of focus and economy of language may have made potential questioners shy. His response to a question on the role of intellectual property in stem cell research was remarkable for its understatement: “Going forward in iPS cells, it will be an interesting [patent] landscape for years to come. It will be a long time before the dust settles”. When another audience member asked whether the Bush administration restriction on federally-sponsored research to the existing UW stem cell lines had been an economic boon for Wisconsin, he laughed and conceded, “Actually, yes”. (In doing so, UW became established as the site for WiCell and the National Stem Cell Bank, distributing the lines to hundreds of scientists worldwide and training them in their use.)
The most surreal moment of the event was when the UW mascot Bucky Badger brought out a birthday cake to Dr. Thomson and the audience sung “Happy Birthday”. The look on his face was a mixture of bemusement and mild horror. Cute antics like this were clearly not his bag.
The free birthday cake served to everyone afterwards, however, was delicious.
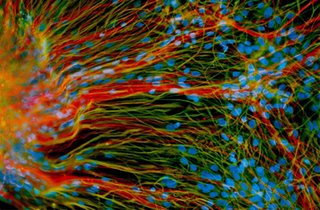
Image of precursor neural cells derived from human embryonic stem cells is from the laboratory of Su-Chun Zhang, University of Wisconsin, and made available through www.stemcellresources.org. Precursor neural cells shown in this image are growing in a lab dish and generate mature neurons (red) and glial cells (green).
Labels: science event, stem cells


2 Comments:
Karren Jeske sends the following:
Great post and New Year's resolution, Robyn. Madison will be all the richer with your attendance. Your readers may be interested in a Milwaukee Journal Sentinel series on stem cells. Part II can be found at: http://www.jsonline.com/features/health/36146249.html
Good news for Thomson.
UW's Thomson wins prize, could be on track for Nobel
By Mark Johnson and Kathleen Gallagher of the Journal Sentinel
Posted: Dec. 18, 2008 11:26 a.m.
University of Wisconsin-Madison scientist James Thomson is among a group of stem cell researchers who have won the 2008 Massry Prize, the university said this morning. Eight of the recipients of the prize have gone on to win the Nobel Prize.
A decade ago Thomson became the first scientist to successfully isolate and culture human embryonic stem cells, and last year was one of two scientists to figure out how to turn human skin cells into stem cells without using embryos. The other, Shinya Yamanaka of Kyoto University in Japan, was among those who shared the prize with Thomson. Rudolf Jaenisch, of Massachusetts Institute of Technology, also shared the prize.
Organizers of the Massry Prize, which has been awarded since 1996, said the techniques pioneered by the three are already proving in animal studies that the cells made without embryos can be genetically matched to recipients so they're not targets for immune rejection. The "amazing advance" opens new horizons for the application of stem cell technology with great promise for individuals suffering from genetic diseases, prize organizers said in a news release.
"Stem cells have the potential to help us find the root cause of disease, so we can prevent it, to test new drugs at the human cellular level and thereby reduce reliance on animal testing, and to offer regenerative therapies to patients," Thomson said in the release.
Post a Comment
<< Home